Exploration of Current Sapphire Window Production Techniques
Introduction:
Sapphire Windows are transparent optical components made from sapphire (Al2O3) designed to maximize the transmission of a certain interested spectrum. The sapphire material selected to manufacture optical sapphire windows is not a natural sapphire gemstone, but sapphire crystals that are grown using artificial techniques with few contaminants and high purities. Sapphire is an excellent optical material with the broad optical transmission, exceptional mechanical hardness, superior high-temperature tolerance/chemical resistance, and advantageous dielectric properties. (Click here to learn the Basic Properties of Sapphire). Sapphire windows are often utilized in demanding applications of extreme conditions and adverse climates. The applications of sapphire windows are extensive, including:
- Drilling Viewport Windows, Protective Laser Windows, and Gun Sights
- High-temperature Plasma Chambers and Combustion Chambers
- High-pressure Protective Windows, Deep Water Windows, Corrosion-resistant, Watch Glass
- Phone screens, Fingerprint Identification Windows
In this article, we will be introducing the manufacturing process of optical sapphire windows, including the growth of sapphire crystals, the cutting process and orientations of sapphire windows, and further grinding, polishing, and washing/cleaning techniques to finish the entire manufacturing procedure of sapphire windows.
Figure 1. Examples of Stocked Sapphire Windows from Shalom EO.
Bulk Single Crystal Sapphire Crystal Growth
The procurement of bulk sapphire crystals is the prerequisite for the production of sapphire windows. The dimensions and qualities of the bulk sapphire crystal have to conform to definite criteria according to the requirements of users. Poor qualities of sapphires could lead to severe problems in the sapphire window (e.g. bubble inclusions in the sapphire could cause undesired optical absorption presenting in the window and lattice dislocations might cause depositions during the polishing process of the sapphire window). Therefore sapphire window manufacturers should be careful while fabricating or purchasing raw sapphire materials.
Sapphire is a corundum composed of Aluminum Dioxide (Al2O3) arranged in 3D-regular, periodical, and reduplicated crystalline state of hexagonal or cubic structures. Besides Al2O3, nature sapphire contains substantial amounts of a foreign substance, which is the exact cause of the gorgeous color appearing in sapphire gemstones.
There are 9 kinds of sapphire known so far in total, including gamma sapphire, beta sapphire, alpha sapphire, and eta, zeta, theta, kappa, rho, chi sapphire. The sapphire selected to produce sapphire windows is Alpha Single Crystal Sapphire grown using specific artificial techniques in the labs and factories. These artificial sapphires are also often referred to as “Sapphire Glass” (which could be misleading, because sapphire glass is not a real kind of glass, it exists in crystalline forms rather than amorphous glass forms). Compared with natural sapphire, the grown sapphire or sapphire glass is colorless, and features higher Al2O3 purities, exclusion of water, and more ordered, predictable micro-structures, allowing it to fulfill industrial and optical-grade demands better.
The atomic structure of alpha-sapphire used to produce sapphire windows is shown in the figure below. The left half of the figure shows that alpha-sapphire constitutes hexagonal-closest piled oxide floors, and the 3/2 gaps of the octahedron are filled with Al3+ ions. The right part of the figure illustrates six floors of Al2O3 unit cells arranged in ARAB manners. Each floor contains 3 oxide atoms and 18 oxide atoms in total. Regarding the Al atoms, on the 1 and 4 floors, there are two each, and the remaining 4 floors contain 3 each, so that will be 12 Al atoms in total.
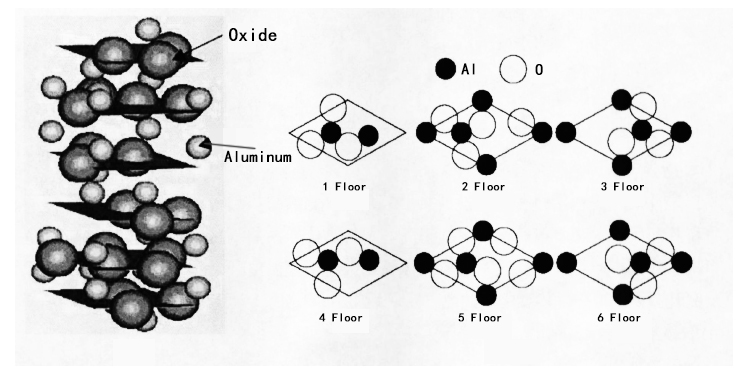
Figure 2. Shows the Atomic Structure of Alpha Single Crystal Sapphire (Alpha-Al2O3)
▶Kyropoulos Sapphire Growth Method
At present, the Kyropoulos Method, or KY Method, in short, is the most common method applied to produce optical-grade single sapphire crystals, so we will introduce it in detail. The method is first contrived in 1926 and was not intended for sapphire growth. In the 1970s, the Soviet Union first applied this method to bulk sapphire growth. After decades of researchers’ continuous transformation and development, now the KY method has become one of the mainstream and advanced approaches to fabricating sapphire crystals of large dimensions and high quality. The method is a modified Czochralski method, the apparatus and the operations of the two are similar, the major difference between the former and the KY method being that the Czochralski method uses uplifting as the major motivation of crystal growth, while the KY method relies on the precise modulation of the temperatures to grow the sapphire crystal.
The steps of the Kyropoulos method are listed below (Note that the growth process is carried out in the chemical-inert atmosphere in nitrogen gas or vacuum):
1. A certain amount of feedstock (the feedstock is Al2O3 of high purities) is put into a crucible inside a KY growth furnace with heaters around the crucible, and the ingredients are melted into a solution of temperatures above 2100°C, which is the melting point of the sapphire feedstock.
2. A rod with the seed crystal attached at the bottom is then dipped into the solution.
3. To let the seed crystal grow, the temperature of the solution should be lower than the melting point of the sapphire. First, adjust the heater so that the temperature at the interface of the seed crystal and solution is a slight percentage higher than the melting point. Lift the seed crystal a bit to pull out the initial head sector of the sapphire crystal.
4. The remaining part of the crystal is formed using a gradual and slow cooling process of the solution. Heaters need to adjust with caution to make the temperature gradient of the crystal appropriate, preventing excessive thermal stress. The diameter of the sapphires grown could be almost equal to the diameter of the crucible. During the crystal growth, the rod attaching the seed crystal could be rotated to improve the temperature distribution. A moderate up-lifting might also be performed at suitable growth phases to expand the heat-radiation area.
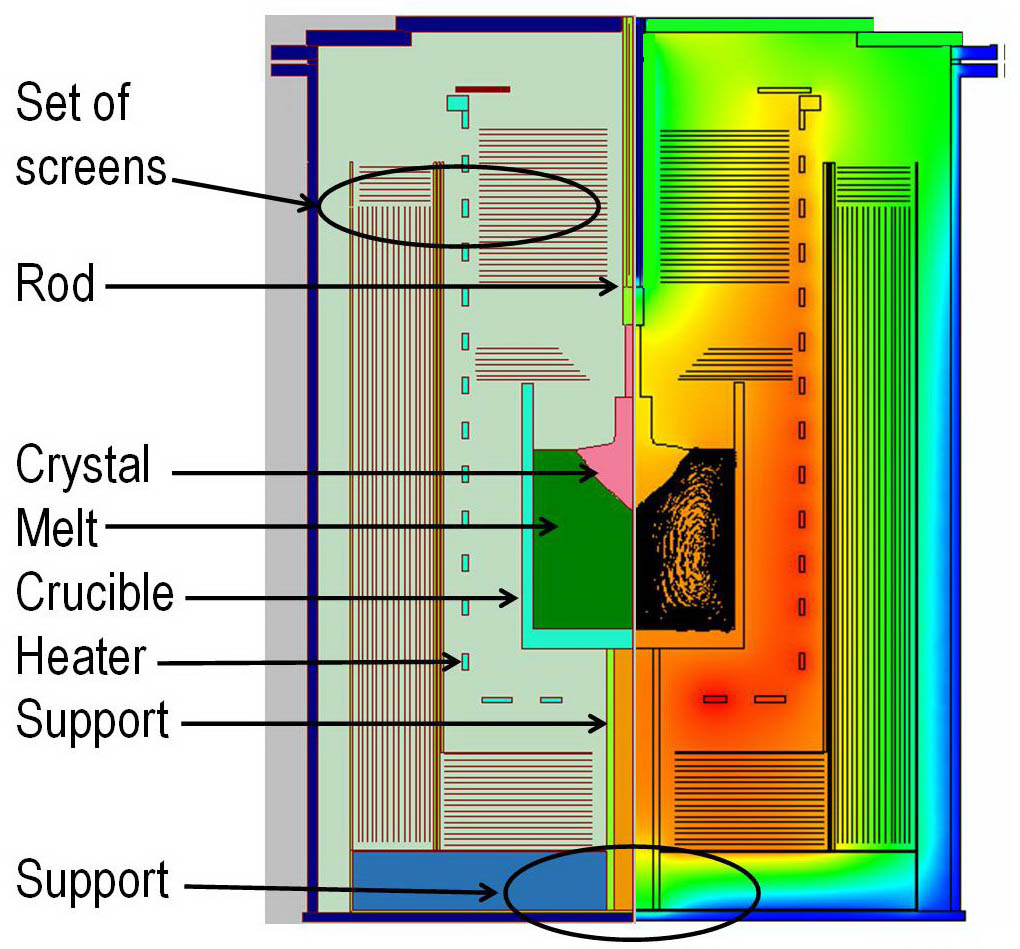
Figure 3. The Fabrication apparatus of Kyropolous Sapphire Growth
▶Evaluation of The Kyropoulos Method
The Kyropoulos method is developed based on the Czochralski method, so it inherits the advantages of the Czochralki method like short growth timescale and high integrities of the sapphires grown. While compared with the Czochralski method, the KY method exhibits further superiorities.
Utilizing the KY method, gigantic bulk sapphire crystals with diameters greater than 100mm could be obtained without obstacle during the shouldering stage, whilst it’s somewhat difficult in the case of the czochralski method. There is little lifting up during the growth phase of sapphire, so the temperature gradient could be modulated with higher precision, minimizing the densities of lattice dislocation and thermal stress. The KY method is also more beneficial for fabricating sapphire crystals with designated orientations.
However, there are defects within the Kyropolous methods. One of them is the exorbitant cost of temperature controllers. The dissipation of the feedstock is also higher than that within the SAPMAC method.
▶Other Methods of Sapphire Growth
When the first synthetic sapphire came into being in 1902, the chemist Auguste Verneuil invented a Verneuil Method using flame fusion to produce sapphire. Al2O3 powders are added to the OH flame and the melted drops coagulate on a cooled seed rod. This method, now, however, has become obsolete because it is difficult to modulate the heat of the flame hence causing fractures on the end product, and quite a percentage of Al2O3 will be depleted in the flame fusion process, increasing the production cost.
In 1967, Dennis and Fred Schmid devised a Heat Exchange Method for sapphire growth. The working principle of this method is to manipulate the temperature gradient of the melted feedstock solution inside the crucible using a graphite heater and a heat exchanger releasing nitrogen, and sapphire will be fruited on the seed crystal placed in the center of the bottom of the crucible. This method has proven successful in fabricating large-dimension sapphires and because there is no stirring of solution causing turbulence during the course, end products are of high homogeneities. However, this method is not time-effective, it might take one week to produce a 320mm diameter sapphire. Besides, the heat exchange method could not be utilized to grow c-axis sapphires.
The Czchoralski Method is almost identical to the Kyropoulos method with the exception that sapphire crystals are formed outside the crucible using an upward lift-and-pull instead of a gradual and mild cooling of the solution inside the crucible. This method is now often substituted by the KY method in sapphire growth because of its limit in fabricating large-dimension sapphires and the negative factor that substantial thermal stress might occur in the sapphire during the lifting course. Also, this method has trouble producing large-diameter sapphires greater than 20mm, because a natural cleavage plane will be introduced down the center of the sapphire ingot when using this method.
The SAPMAC Method is another novel technique developed based on the Kyropoulos method and the Czochralski method. In contrast to the two, the SAPMAC technique involves a tinier amount of lifting-ups and more delicate temperature modulation divided into segments corresponding to the growth phase of the sapphire.
Other methods of sapphire growth include Edge-defined Film-fed Growth (EFG), Temperature Gradient Technique (TGT), Horizontal Directional Crystallization, etc.
Manufacturing Sapphire Windows
The manufacturing of Sapphire Windows constitutes a series of complex production stages encompassing drilling, radial-grinding, cutting/slicing, chamfering/beveling, axial-grinding, polishing, and washing/cleaning. These main production stages are summarized below. Some critical production stages including cutting/slicing, tangential grinding, and polishing are explained in more detail.
▶Drilling of Sapphire Windows
Sapphire rods are first gouged out from the bulk single crystals according to the diameters of the sapphire windows. The outlines of the sapphire rods are also different as the window shape varies (e.g. If the final product is a rectangular sapphire, then the sapphire rod should be a cuboid). The gouging tool is often a tube-shaped diamond knife that cuts into the bulk sapphire and sapphire rods are obtained inside the hollow chamber of the diamond knife. On some occasions the bulk sapphire is grown in accordance with the diameters of the window and this step could be skipped.
▶Radial Grinding of Sapphire Windows
It is possible that the side profile of the bulk sapphire or the sapphire rods is not in the ideal precise geometric conditions and the side faces might be too rough and uneven. Grinding wheels with abrasive particles on the grinding front are often hired to grind the trunk of the sapphire rods along the radial direction of the rod.▶Cutting/Slicing and The Orientations of Sapphire Windows
The orientation of a crystal is a vector describing a random line connecting two nodes on the lattice. Due to the anisotropic nature of crystals, the distribution and the arrangement manner of the atoms change along different directions or upon different lattice planes. The result of this is that the properties and behaviors, even of the same crystals, but with different orientations will differ to a significant extent. This is the reason of choosing the proper orientations and cutting planes of crystals is critical when using crystals to produce various components and elements.
The Sapphire crystals utilized to produce sapphire windows of different functions will be grown and cut/sliced with an engineered orientation, and the orientation is often determined because it will optimize the crystal’s performance to achieve the intentions of interest.
The lattices within the sapphire are arranged in a hexagonal structure. When a sapphire element is produced, the direction of its inner architecture affects the functionalities of the element.
The common orientation of sapphire windows is “a”, “c”, “n”, and “m”, as shown in the figure below.
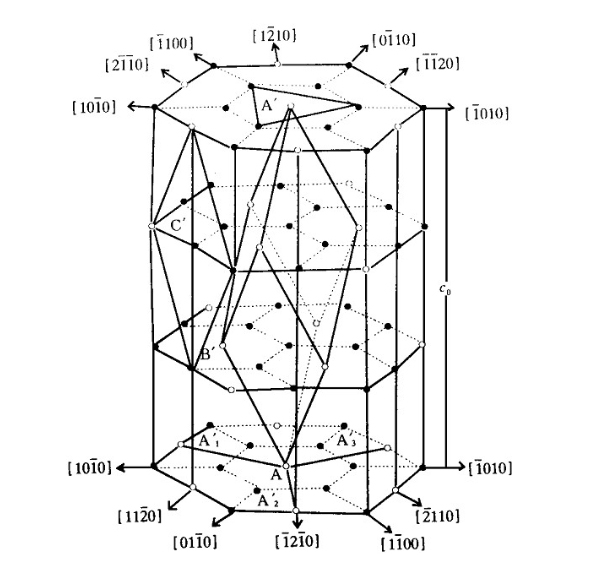
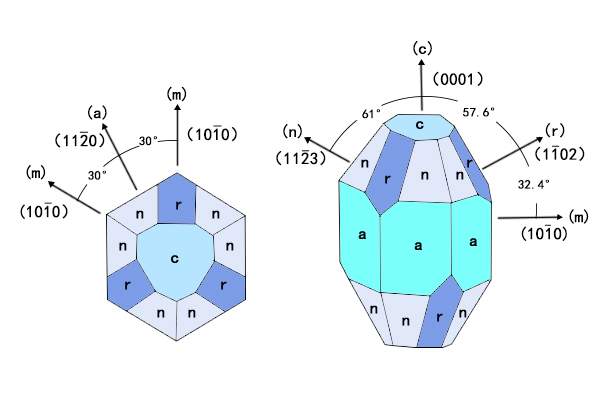
Figure 4.The Lattice Structure of Sapphire and Common Sapphire Crystal Orientations
The c-cut and a-cut are the two orientations leveraged most in optical and industrial applications. The orientation index and the characteristics of c-cut and a-cut are listed below.
C-cut: Orientation index (0001). The sapphire is cut in a direction perpendicular to the c-axis. The c-axis is the optical axis of sapphire. light projected along the direction of the optical axis will not encounter birefringence. In practice, the light will be incident with a perpendicular angle to the apertures of the sapphire windows, which means the light will travel inside the sapphire windows parallel to the optical axis, eliminating the birefringence effect. C-cut Sapphire Windows are often chosen for critical optical missions (e.g. laser windows). C-cut sapphire is sometimes called zero-degree sapphire, or z-cut sapphire.
A-Cut: Orientation index (11-20). A-cut means the sapphire crystal is cut along the direction perpendicular to the a-axis. A-cut Sapphire Windows contain exceptional mechanical hardness and scratch resistance, hence are considered the most pertinent kind of sapphire windows for protective and packaging usages such as the sight glass of wristwatches.
There are also Random-cut Sapphire Windows. Random-cut implies the element is cut or sliced with no specific regard to directions, it might contain any orientations. This orientation is common because of lower costs and is acceptable if there are no stringent requirements about optical or mechanical qualities. However, as mentioned above, because the behavior of sapphire varies depending on orientations, random orientation might be subject to spontaneous variations of strength and other properties in the final product.
Before the cutting or slicing procedure, sometimes the manufacturer would grow the bulk sapphire crystal of a specific orientation. For example, the bulk sapphire designated to produce a c-cut sapphire window will be grown with an orientation that maximizes the utilization efficiencies of the c-planes.
Sapphire is the second hardest material on earth, add to the fact its strong structural strength, and efficient cutting and slicing of sapphire propose challenges to manufacturers. The cutting tool must be powerful enough the conquer the hardness of sapphire, at the same time quick in cutting with minimized wastage of the raw material, and acceptable in price.
The mainstream technique in the current market to cut and slice sapphire windows is Diamond-wire Cutting. The diamond wire saw is often made from metal wires electro-plated with diamond abrasives. The common cross-sectional diameter of wire is 0.12-0.5mm, while diameters below 0.1mm are sometimes attained to deliver tinier cutting kerfs and produce thinner windows. During the cutting operation, the diamond wires are either wound on a spool that rotates at high speed and makes the wire saw move back and forth or coiled into a loop shape.
The diamond-wire cutting technique proved to be a reliable approach for realizing low cutting loss and large-aperture cutting. The variations of diamond wire cutting include single wire-diamond cutting and multi-wire diamond cutting which can realize simultaneous cutting of a batch of sapphire window substrates. Regarding the abrasives, diamond wire sawing could also be separated into loose abrasive diamond sawing and fixed abrasive sawing.
▶Chamfering/Beveling of Sapphire Windows
The chamfering or beveling process refers to the profiling of the elements’ sharp edges into slopes of certain angles. This is because the sharp edges are prone to chips (i.e the peeling-off of little pieces from the elements) that impair the elements, while the slopes are less prone to chips. This is a protective measure hired in the production of a lot of optics, including sapphire windows.
▶Axial Grinding of Sapphire Windows
After slicing, the axial planes of sapphire blanks (which are the functional planes of sapphire windows) will be ground. This procedure aims to shape the element into the appropriate thickness, eliminate the scratches and defects on the functional planes, reduce the roughness and elevate the flatness until the sapphire substrates are prepared for polishing.
The grinding techniques for single crystal sapphire windows are manifold, including Mechanical Grinding, Chemical-Mechanical Grinding (PCG), ELID, etc. Sapphire is a brittle and stiff material with extraordinary hardness, so it would be difficult to balance strength and precision during the grinding course.
1)Mechanical Grinding of Sapphire Windows:
The traditional grinding technique is mechanical grinding. It uses fluid abrasive slurries, consisting of abrasive particles of greater hardness poured onto grinding pads to exert mechanical forces onto the sapphire elements to remove the excessive sapphire. As the grinding pad spins, the fluid slurries come into contact with the sapphire substrate and the particles inside perform a powerful mechanical removal. Common abrasive slurries are made from W40 boron carbide + W7/W10 boron carbide as the abrasive slurries. Boron Carbide, with molecular formula BC4, is also known as “black diamond”, and is one the hardest materials known (the Mohs index of BC4 is 9.5, while sapphires' is 9). The densities and the sizes of the abrasive particles could be modified according to different precision and speed standards. In the initial grinding phase, coarse-grain grinding slurries are chosen, and later in a finer grinding and lapping stage, fine-grained grinding slurries will be used. Apart from the slurries, no chemical solutions are added, just ionized water is applied to flush the removed sapphire materials down.
Along with the rapid growth of sapphire-based technologies, ultra-thin and high-precision sapphire windows also embrace growing demand. This poses big challenges to the traditional grinding techniques of sapphire elements. As this pure-mechanical method uses abrasion particles of greater hardness than sapphire, the material removal strength could be too vigorous, causing unpredictable scratches and minor injuries, lowered surface qualities and concentration of stress within the sapphire substrates, affecting the live span of the sapphire windows.
2)Chemical Mechanical Grinding of Sapphire Windows
A modification to the aforesaid pure-mechanical method is Chemical Mechanical Grinding (CMG). This method also uses fluid grinding slurries, but the abrasive particles are made from materials of similar hardness to sapphire, such as corundum, SiC, etc. Since the abrasive particles are of similar hardness to sapphire, chemical grinding solutions must be utilized in conjunction to strengthen the material removal. Strong, corrosive acid or alkaline solutions are chosen. The acid or alkaline weakens the covalent bond of sapphire atoms on the front surface of sapphire substrates and forms a softened section, then mechanical forces remove the softened section, realizing material removal. This CMG technique provides more delicate grinding controls and helps to obtain smoother sapphire window surfaces.
However, the consumption of slurries leads to rising production costs, redundant slurries also derive environmental contamination. Besides, in the grinding course, the loose abrasives in the slurries move in spontaneous manners, it’s difficult to control the grinding course, and loose particles might cause unpredictable damage to the sapphire windows’ surface.
3)Fixed Abrasive Chemical Mechanical Grinding of Sapphire
In recent years, multiple innovations have been developed in the market in response to these problems. One of them is Fixed Abrasive Chemical Mechanical Grinding (FA-CMG), the abrasive particles are fixed with the grinding pad instead of moving in a loose manner within the grinding fluid. One approach of FA-CMG is using grinding plates embedded with diamond particle grids, in combination with a grinding lubricant composing ionized mixed with 2% ethanediol. The ethanediol composition helps to increase the activeness of the surface of sapphire substrates, softening the contact interface, enhancing the material removal speed, and improving the surface qualities. The major advantage of this approach is the reduced uncertainties attributed to the fixed abrasion particles. And because the abrasive particles have been planted into the grinding plate, additional grinding slurries are not needed, saving the production cost.
4)Fixed Soft Abrasive Grinding (FSAG) of Sapphire
There is also the Fixed Soft Abrasive Grinding (FSAG) technique. Compared with FA-CMG, in the fixed soft abrasive grinding technique no liquid form chemical solutions are added during the grinding course, and the abrasive particles are made from materials softer than sapphire (e.g Silicon Dioxide, Magnesium Oxide). Removal of sapphire is realized based on a solid phase chemical reaction between the sapphire window substrate and the fixed abrasives. According to the chemical, and physical properties and the micro-structures of single crystal sapphire, using specific abrasives (to minimize the potential damage, the hardness of the abrasive must be pertinent) that makes chemical reaction with sapphire (and the product of chemical reactions can be removed at ease). The operation details are stated as follows:
Mixtures of SiO2 or MgO, Fe2O3 particles, phenolic resin, NaCO3, and KmnO4 are pressed into a batch of circular abraser pills, the abraser pills are then distributed with caution on a grinding disc that rotates. The SiO2 and MgO, Fe2O3 particles are the main contents, making chemical reactions with sapphire under certain conditions, the phenolic resin and NaCO3 are the bonding agents, increasing the porosity and self-sharpening characteristic of the abraser pills, and KMnO4 is the active agent which helps to promote the reaction speed.
As the grinding disc rotates, a great amount of heat is generated at the contact area between sapphire and abraser pills, leading to a substantial temperature rise reaching the solid phase chemical reaction temperature of the substances. Meanwhile, the active and bonding agents within the abraser pills keep spreading boosting and PH modulation doses onto the front face of the sapphire, thus solid-phase chemical reactions are triggered between the soft abraser pills and sapphire window substrate. The result of the chemical reaction is a softened portion at the interface. The softened portion is lower than the sapphire and could be easily removed. The abrasive particles then perform moderate, microscale mechanical material removals. Because the abrasive materials are softer than sapphire, the danger of surface damage plummets. Furthermore, this fixed soft abrasive grinding technique does not use grinding slurries and chemical grinding solutions, which helps to cut the production cost down and reduce environmental harmfulness.
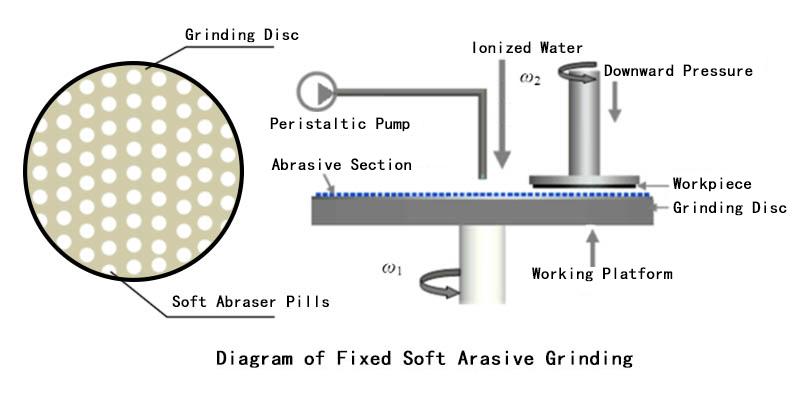
Figure 5. Set of Fixed Soft Abrasive Grinding
4)ELID (Electrolytic In-process Dressing) Grinding of Sapphire Windows
There is also an ELID (Electrolytic In-process Dressing) sapphire grinding technique utilizing a diamond grinding wheel and the electrolyzation effect. In the grinding process, the surfaces of the diamond abrasives on the diamond grinding wheel are continuously refreshed by the electrochemical reaction. ELID grinding technique is often adopted to produce ultra-high-precision sapphire windows.
▶Polishing of Sapphire Windows
The polishing technique of sapphire can be divided into Single Side Polishing (SSP) and Double Side polishing (DSP). Regarding the flatness (TTV) and warp (WARP) of sapphire, in general, the sapphire window substrates produced using double-side polishing equipment are better than the sapphire wafer produced using single-sided equipment, which is attributed to the principle of double-sided polishing. In the double-side polishing procedure, the sapphire windows go under both self-rotation and large-diameter revolutions, so that higher flatness could be obtained. Furthermore, the front and back sides are subject to equal stress and synchronous polishing progress, Therefore, the curvature of the sapphire wafer is also better.
The composition of the polishing solution is another critical element of great influence. At present, the mainstream of sapphire polishing is using nanoscale Silicon Dioxide (SiO2) as the polishing solution, the achieved roughness of the final polished surface could be below Ra 0.3nm in general. In the polishing process, the reaction between the SiO2 polishing solution and sapphire is shown in the following formula: Al2O3+2SiO2+2H2O=Al2Si2O7·2H2O. However, along with the exponential growth of demand for sapphire windows, manufacturers and researchers seek to innovate the traditional SiO2 sapphire polishing method in pursuit of a greater polishing rate and higher precision.
There has been developed the Aluminum Oxide Polishing Method, which uses Al2O3 abrasive particles as the main composition of the polishing solution. Yu Jiangyong and Liu Yuling from Hebei, added nanoscale Alumina particles (with a concentration of 2%) to the SiO2 polishing solution and saw an evident increase in the polishing rate of sapphire from 9 μm/h to 11.3 μm/h, and The dilution ratio of the SiO2 polishing solution could be lowered from 1:1 to 1:2. Nevertheless, the aluminum oxide abrasives produced are often fabricated through a complicated procedure including calcination, grinding and screening, obtaining aluminum oxide with uniform and nano-scale particle sizes is difficult and expensive. The most severe flaw is that the hardness of aluminum oxide is high, the polished sapphire windows are often scratched and defective, and the viscosities of the polished surface are large, leading to washing difficulties, and it is hard to ensure the surface flatness of the polishing material. Another significant obstacle to the dissemination of the aluminum oxide polishing technique is the high requirements for the rotation speed of the equipment. Therefore, using Al2O3 as a polishing liquid is still at the experimental stage at present.
Researcher K. Bakshi and his companions from the United States utilized alpha or beta silicon carbide polished sapphire with an average particle diameter of 100-400 nm. He first softened the silicon carbide surface with a silica coating in an attempt to reduce surface scratching on the sapphire. The reason is that the silica coating can passivate sharp corners of the silicon carbide particles. The core of the particle is still silicon carbide with higher hardness, and the abrasive maintains a better polishing removal rate. He also incorporated a composite polishing solution of silicon carbide and silicon dioxide (30% SiC, 70% SiO2) to polish R-orientation sapphire, and the polishing rate can be increased 1.3-1.5 times greater than SiO2 polishing liquid without adding SiC.
Zong Simiao and Liu Yuling, from China, utilized a polishing solution self-developed in their lab with a higher pH value of about 12.5, and a high abrasive concentration (50%), under a rapid temperature rise to 45 °C. The polishing rate of sapphire can reach 11.3 μm /h. Li Shurong and Jin Zhuji from Dalian studied the effect of different pH control agents on the polishing rate, comparing four inorganic bases, including NaOH, KOH, Ca(OH)2, and Ammonia, and found that KOH can improve to a significant extent the sapphire polishing rate. Researchers also found that adding a suitable amount of Fe-Nx/C activator agents to the SiO2 polishing solution also improves the polishing rate (The Fe-Nx/C activator contains Fe2O3, Fe3O4, and nitrogen).
Apart from the polishing solution, the polishing apparatus also has a great impact on the polishing rate. Leveraging the Vibration of Ultrasonic Wave can elevate the removal rate (at about 3.8 times) in the polishing course of sapphire with SiO2 solution, and the roughness could be reduced too. Possible reasons are: (1) The motion path of silica particles on the sapphire surface is lengthened; (2) The pressure between the interface of SiO2 particles and sapphire is increased. Therefore the vibration and cavitation of ultrasonic waves strengthen the effect of silica particles on the surface of the sapphire. In practical operation, ultrasonic is applied to the polishing head of the single-sided polishing machine.
In addition, the orientation of sapphire windows is also an essential factor in the polishing rate. In general, the polishing rate of A, M, and R orientation is slower than that of C-cut sapphire. This is because the orientation index of C-cut sapphire crystal is (0001), and the atomic bonding structure is O-Al-Al-O-Al-Al-O, whilst the orientation indice of M and A cut sapphires are (10-10) and ( 11-20), their chemical bonding structure is Al-O-Al-O. The Al-Al bond is weaker than the Al-O bond, and the polishing of C-cut sapphire encounters the Al-Al bond in the main, so the polishing rate of c-cut sapphire is faster in comparison.
In real-life production workshops, the polishing precision requirement of A-cut sapphire windows is often not high. SiO2 polishing liquid and non-woven fabrics will work well. Whilst in the case of c-cut sapphire windows higher precision is often required. Polishing accessories from Universal Optical are prevalent, with the removal amount of polishing reaching 10 μm/90min.
▶Washing and Cleaning of Sapphire Windows:
After SiO2 polishing, a section of a foreign substance containing Al2SiO5 in the main is generated upon the sapphire substrate front as a result of the chemical reaction between silicon dioxide, sapphire, and water. Therefore washing and cleaning are required to dislodge the foreign substance and the polishing scraps.
The cleaning process after sapphire polishing is complicated. The industrial mainstream technique is Wet Bench Cleaning, and there are various kinds of chemical reagents. The standard cleaning process most uses SC1 (ammonia, H2O2, water), SC2 (HCL, H2O2, water), and Sulfuric acid + Phosphoric acid. The proportions of the several components in SC1 and SC2 can be adjusted according to the actual situation. While the ratio of sulfuric acid to phosphoric acid is often 3:1. Regarding the cleaning quantities, the most common measure is multi-piece cleaning (Cassette Cleaning), with about 25 pieces cleaned in one round. The SC1 cleaning tank is equipped with ultrasonic wave generators, and the standard frequencies of the ultrasonic wave are 40 kHz, 80 kHz, 120 kHz, and 200 kHz.
Tags: Exploration of Current Sapphire Window Production Techniques

