ZnSe (Zinc Selenide)
ZnSe is used widely for IR components, windows and lenses, and for spectroscopic ATR prisms. Zinc Selenide is one of the materials of choice for CO2 laser optics operating at 10.6 microns.
Physical and optical properties
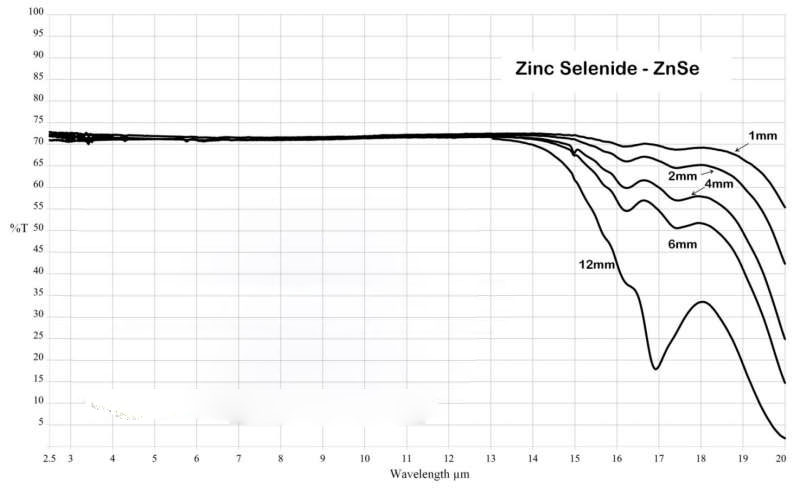
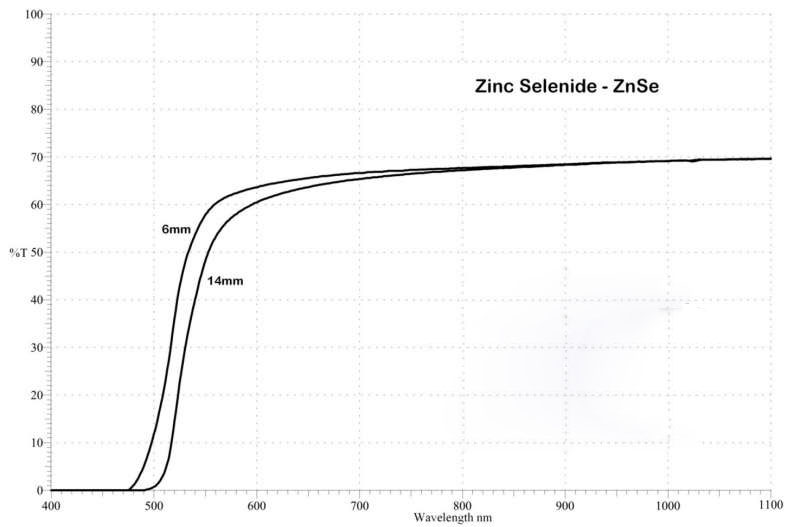
| Transmission Range : | 0.6 to 21.0 μm |
| Refractive Index : | 2.4028 at 10.6 μm |
| Reflection Loss : | 29.1% at 10.6 μm (2 surfaces) |
| Absorption Coefficient : | 0.0005 cm-1 at 10.6 μm |
| Reststrahlen Peak : | 45.7 μm |
| dn/dT : | +61 x 10-6/°C at 10.6 μm at 298K |
| dn/dμ = 0 : | 5.5 μm |
| Density : | 5.27 g/cc |
| Melting Point : | 1525°C (see notes below) |
| Thermal Conductivity : | 18 W m-1 K-1 at 298K |
| Thermal Expansion : | 7.1 x 10-6 /°C at 273K |
| Hardness : | Knoop 120 with 50g indenter |
| Specific Heat Capacity : | 339 J Kg-1 K-1 |
| Dielectric Constant : | n/a |
| Youngs Modulus (E) : | 67.2 GPa |
| Shear Modulus (G) : | n/a |
| Bulk Modulus (K) : | 40 GPa |
| Elastic Coefficients : | Not Available |
| Apparent Elastic Limit : | 55.1 MPa (8000 psi) |
| Poisson Ratio : | 0.28 |
| Solubility : | 0.001g/100g water |
| Molecular Weight : | 144.33 |
| Class/Structure : | HIP polycrystalline cubic, ZnS, F43m |
No = Ordinary Ray
µm |
No |
µm |
No |
µm |
No |
0.54 |
2.6754 |
0.58 |
2.6312 |
0.62 |
2.5994 |
0.66 |
2.5755 |
0.7 |
2.5568 |
0.74 |
2.5418 |
0.78 |
2.5295 |
0.82 |
2.5193 |
0.86 |
2.5107 |
0.90 |
2.5034 |
0.94 |
2.4971 |
0.98 |
2.4916 |
1.0 |
2.4892 |
1.4 |
2.4609 |
1.8 |
2.4496 |
2.2 |
2.4437 |
2.6 |
2.4401 |
3.0 |
2.4376 |
3.4 |
2.4356 |
3.8 |
2.4339 |
4.2 |
2.4324 |
4.6 |
2.4309 |
5.0 |
2.4295 |
5.4 |
2.4281 |
5.8 |
2.4266 |
6.2 |
2.4251 |
6.6 |
2.4235 |
7.0 |
2.4218 |
7.4 |
2.4201 |
7.8 |
2.4183 |
8.2 |
2.4163 |
8.6 |
2.4143 |
9.0 |
2.4122 |
9.4 |
2.4100 |
9.8 |
2.4077 |
10.2 |
2.4053 |
10.6 |
2.4028 |
11.0 |
2.4001 |
11.4 |
2.3974 |
11.8 |
2.3945 |
12.2 |
2.3915 |
12.6 |
2.3883 |
13.0 |
2.3850 |
13.4 |
2.3816 |
13.8 |
2.3781 |
14.2 |
2.3744 |
14.6 |
2.3705 |
15.0 |
2.3665 |
15.4 |
2.3623 |
15.8 |
2.3579 |
16.2 |
2.3534 |
16.6 |
2.3487 |
17.0 |
2.3438 |
17.4 |
2.3387 |
17.8 |
2.3333 |
18.2 |
2.3278 |
Zinc Selenide is produced by synthesis from Zinc vapour and H2Se gas, forming as sheets on Graphite susceptors. Zinc Selenide is microcrystalline in structure, the grain size being controlled to produce maximum strength. Single crystal ZnSe is available, but is not common but has been reported as having lower absorption and thus more effective for CO2 optics.
Zinc Selenide oxidizes significantly at 300°C, exhibits plastic deformation at about 500°C and dissociates about 700°C. For safety, Zinc Selenide windows should not be used above 250°C in normal atmosphere.
Tags: ZnSe (Zinc Selenide)

